"
And Tesla has had battery fires," he said, quietly.
FWIW, I read up on Lithium-ion chemistry/technology ages ago. I like to know about the insides of the tools I have and the technology I use. I've worked in high-tech all my adult life (apart from very recently).
Lithium-ion cells are relatively difficult to make, difficult to deploy safely (compared to earlier types of cell), and pretty environmentally unfriendly. To do the parallel cell thing you have to do
even more cell matching than normal with other chemistries, and it IS more likely to fail as cells age (because they're not used identically in that situation, especially in automotive applications). There are several Wikipedia paragraphs on
the current Tesla arrangements. That one is fairly concise. Note the last sentence: "
The liquid-cooled battery pack uses an intumescent gel to aid in fireproofing and even heat distribution." Tesla only succeed in this approach because they modularize - building the big battery from individually-managed modular batteries, and the management system has to be complex and comprehensive.
Regarding voltage, all types of cells fluctuate between fully charged and safe (recoverable) levels of discharge. Lead-acid cells are
nominally 2V, but at full charge (the level at which minimal sulphonation takes place and electrolysis is just starting, a healthy cell measures around 2.2-2.3V on float (trickle) charge; 1.95V when recoverably discharged.
2V is used as a number because it's the voltage you design-to when using the cells. So at 12V (6 cells), and using an appropriate power drain characteristic for the battery design, you get the power delivery life specified by the manufacturer, and can say "It lasts x hours/minutes on one charge". If you used 2.2V as the standard, your "battery life" between charges would be measured in seconds (it's the capacitance effect rather than electrochemistry, I suspect, that allows the higher voltage to stay when the charger is switched off).
Lithium-ion cells may well reach 4V when fully charged, but nominally range from 3.3V to 3.7V (as lead-acid are 2V). The fact you can get to 4V is irrelevant - you can't design a practical circuit to take advantage of this (except perhaps in a rail gun in the tropics!). Here's a telling graph:
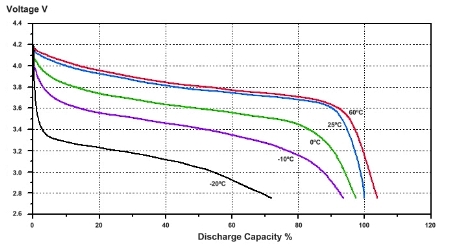
Note the temperature sensitivity - the voltage drops off very dramatically with temperature decrease. And it makes it clear: 3.7V is present at roughly 50% cell capacity at room temp (interpolated, and for the design illustrated). I picked that specific graph because the temperature drop-off is severe and illustrates the point well. There's other good stuff on that page.
This reminds me a great deal of the way manufacturers started to rate audio amplifiers, mainly for the car audio market (it later crept into home HiFi specs too). For decades, amps were measured by the power they could dump into 8 Ohms resistive load, and very occasionally 4 Ohms (2 Ohms for valve amps - I'm old enough!). The main reason for 8 Ohms was that it allowed the speaker manufacturers some leeway in drive unit and crossover designs (XY, are you out there?

).
That de-facto standard was simple and allowed easy, objective comparisons, BUT... it also requires meaty power rails in the amp to overcome the 8 Ohms (current squared times resistance). There's only 12V available for car amplifiers' power rails using simple designs, and that means a practical limit of 36W RMS per amp channel at 4 Ohms).
You can't beat physics! But you can lie, so we got "Peak Mean Power Output" (whatever that means) and sometimes just "Peak" power output. But since 72W RMS in a car is LOUD, the punters were/are none the wiser (and anyway the enthusiasts use 2 Ohm speakers, bridging modes and really heavy speaker cable to get round the issue).*
Using 4V/cell to give the number to put on your kit is just like the "pick a number" approach of the car audio spivs. I'd treat any manufacturer doing it with suspicion.
And finally, quoting Tesla's use of commodity cells is all very well: everyone else using Lithium Ion in high power applications (including Boeing, admittedly with problems too) uses bigger cells and much simpler battery designs. Tesla does gain some really useful things from the approach, odd-shaped battery packs being probably the most important, along with commodity pricing, but they need very complex battery management.
E.
*And of course, you can put in voltage doubling power supplies, etc (the good stuff has this), but it isn't cheap. My point was about basically lying about the numbers, not the actual designs.


































