In another thread concerning tempering tool steel in an oven I gave out a bit of misinformation in error concerning the use of a sand heatsink and that it would raise the Temps in a domestic oven to above the normal range. I was gently enlightened that a heat sink can't raise the Temps. I have no reason to disagree and I am always happy to learn but it does puzzle me.
The original thread is here : making-a-brass-infill-plane-hattori-hanzo-dp-t120331.html
Rather than clutter that excellent build thread up I thought to raise the question here.
Hattori shows a wheat coloured temper on his O1 steel that seems to indicate an oven temperature of around 200 celcius. Ok no worries there. Most ovens in the uk go to 250.
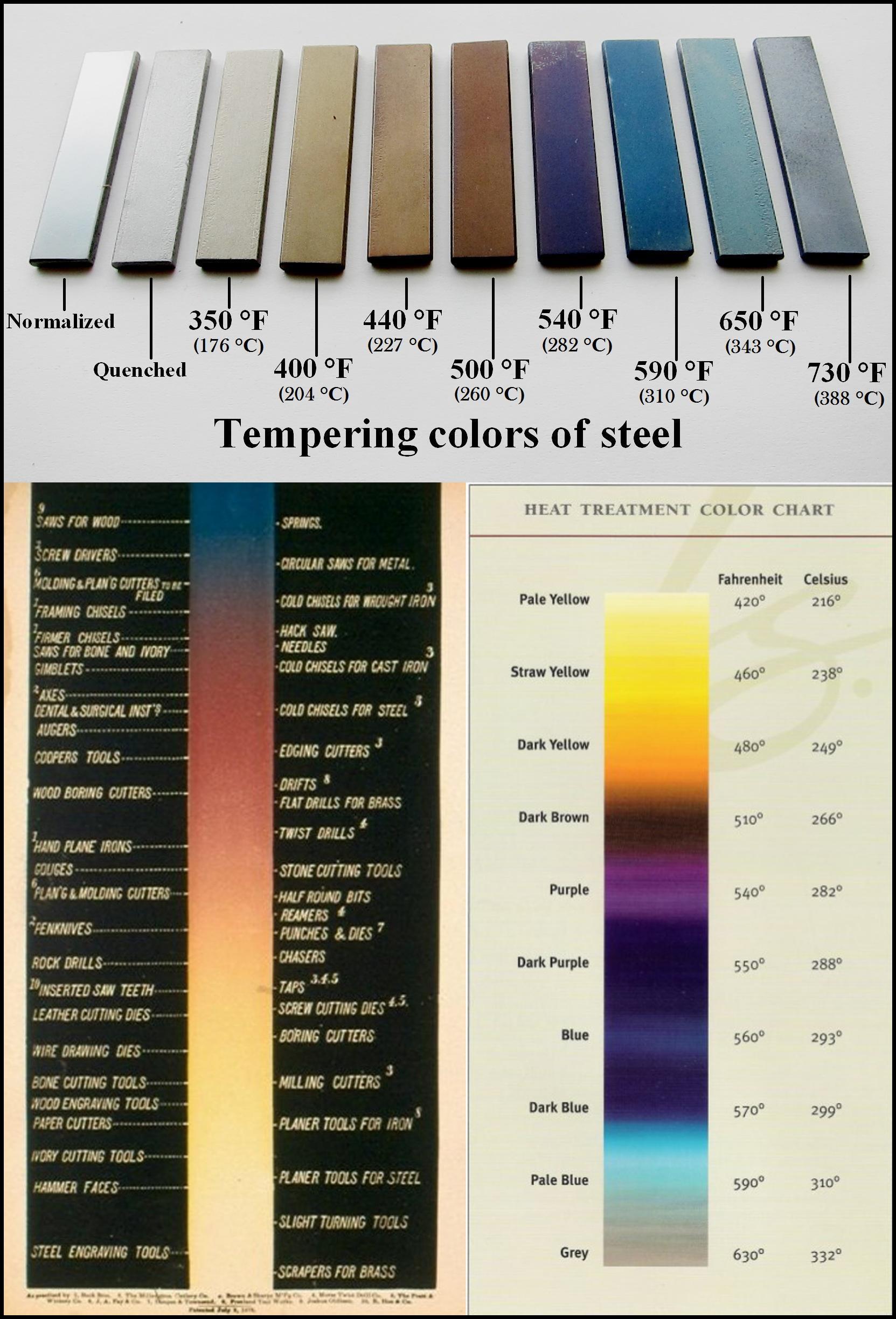
But if the heatsink (sand in a turkey baking foil tray) doesn't actually raise the Temps, then when I did this 4mm thick iron for my first plane, how did achieve this blue colour that grades somewhere between 300 and 330 celcius?

I'm genuinely intrigued.
With this iron actually, I fluked. In use it holds an edge at least as long as as any I have bought. Longer I think. Obviously I'm not applying DW. Type testing conditions! Just seems to work.
Mind you if you ever eaten an animal you killed yourself it's maybe the same delusion as that lol. Fish you catch yourself always tastes better.
Next doors cat too if you can get away with it. :-"
What do we think people?
Cheers
Chris
The original thread is here : making-a-brass-infill-plane-hattori-hanzo-dp-t120331.html
Rather than clutter that excellent build thread up I thought to raise the question here.
Hattori shows a wheat coloured temper on his O1 steel that seems to indicate an oven temperature of around 200 celcius. Ok no worries there. Most ovens in the uk go to 250.

But if the heatsink (sand in a turkey baking foil tray) doesn't actually raise the Temps, then when I did this 4mm thick iron for my first plane, how did achieve this blue colour that grades somewhere between 300 and 330 celcius?

I'm genuinely intrigued.
With this iron actually, I fluked. In use it holds an edge at least as long as as any I have bought. Longer I think. Obviously I'm not applying DW. Type testing conditions! Just seems to work.
Mind you if you ever eaten an animal you killed yourself it's maybe the same delusion as that lol. Fish you catch yourself always tastes better.
Next doors cat too if you can get away with it. :-"
What do we think people?
Cheers
Chris
































